Reportes de Casos
Resultados exitosos de procedimientos quirúrgicos en paciente con trombastenia de Glanzmann
Successful outcomes of surgery procedures in patient with Glanzmann´s Thrombasthenia
Resultados exitosos de procedimientos quirúrgicos en paciente con trombastenia de Glanzmann
Acta Médica Peruana, vol. 40, núm. 2, pp. 146-149, 2023
Colegio Médico del Perú

Esta obra está bajo una Licencia Creative Commons Atribución 4.0 Internacional.
Recepción: 22 Marzo 2023
Aprobación: 22 Marzo 2023
Abstract: We report a 35-year-old female patient with Glanzmann's thrombasthenia (GT) and severe anemia due to abnormal uterine bleeding secondary to uterine myomatosis. She required several admissions of red blood cells and platelet transfusions. An elective subtotal hysterectomy with salpingo-oophorectomy was proposed and recombinant factor VII was required. Surgical and postoperative outcomes were successful, without surgical complications, bleeding, or hemogram alterations. 4 years later, she required tooth extraction because of periodontal disease and pulp necrosis. In Peru, reports of GT patients requiring major and minor surgical procedures are lacking, given the low disease prevalence and the difficulties related to surgery. The report of these successful cases becomes relevant to continue improving GT management.
Keywords: Glanzmann's thrombasthenia, Recombinant factor VII, Hysterectomy, Peru.
Resumen: Presentamos el caso de una paciente de 35 años con trombastenia de Glanzmann (GT) y anemia severa por sangrado uterino anormal secundario a miomatosis uterina. Requirió varias admisiones de transfusiones de glóbulos rojos y plaquetas. Se propuso histerectomía subtotal electiva con salpingo-ooforectomía y se requirió factor VII recombinante. Los resultados quirúrgicos y postoperatorios fueron exitosos, sin complicaciones quirúrgicas, sangrado ni alteraciones del hemograma. 4 años después, requirió extracción dental por enfermedad periodontal y necrosis pulpar. En Perú faltan reportes de pacientes con GT que requieran procedimientos quirúrgicos mayores y menores, dada la baja prevalencia de la enfermedad y las dificultades relacionadas con la cirugía. El reporte de estos casos de éxito cobra relevancia para seguir mejorando la gestión de GT
Palabras clave: Trombastenia de Glanzmann, Factor VII recombinante, Histerectomía, Perú .
INTRODUCTION
Glanzmann thrombasthenia (GT) is an autosomal recessive disorder,[1] linked to platelet membrane defects.[2] GT incidence is estimated at one in 1,000,000 worldwide [1] even though a larger proportion has been described in populations in all of which consanguinity is common.[3] Specific regional studies about GT incidence in Latin America are lacking; nevertheless, a few case series and management recommendations have been published.[4,5]
Based on the membrane glycoprotein (GP) IIb/IIIa complex expression levels, GT is classified in three subtypes: I (GP IIb/ IIIa expression < 5 % of normal), type II (5-20 % of normal GP IIb/IIIa), and III (GP expression is normal, but the protein is dysfunctional).[2]
Clinically, GT is characterized by bleeding, especially mucocutaneous hemorrhage, [1,2] which may be spontaneous or occur after minimal trauma or surgical procedure. Treatment includes the management of bleeding episodes and the prevention of complications. Therapeutic strategies depend on the severity of the bleeding, ranging from local treatment to transfusions.[6] Due to major bleeding risk, GT patients need specific management before, during, and after surgical or invasive procedures.[7] Platelet transfusions and recombinant human factor VII (rFVII) are considered good alternative therapeutic strategies for bleeding and surgical prophylaxis in GT. [2,4-5,8-9]
CASE DESCRIPTION
A female patient, aged 35 years, of Mestizo ethnicity, was admitted into hospital with severe anemia (hemoglobin 4.3 g/dl) associated with abnormal uterine hemorrhage (AUH). She had a history of GT and uterine fibroids, treated with leuprolide and medroxyprogesterone for 2 years. An ultrasound examination showed hemoperitoneum and an emergency laparotomy was performed, after transfusions of 4 red cell packs, fresh-frozen plasma, 2 platelet packs and 2 doses of prothrombin complex concentrate. During surgery, a hemorrhagic corpus luteum cyst was found, requiring an emergency right salpingo-oophorectomy. During follow-up, intramural and subserous uterine leiomyomas were controlled with leuprolide; however, the patient needed several emergency hospitalizations due to severe anemia secondary to AUH, requiring red cell packs transfusions and platelet apheresis. Subtotal hysterectomy plus left salpingo- oophorectomy was proposed as a definitive treatment. During the preoperative screening, anti-human leukocyte antigen (HLA) platelet antibodies were identified and a rFVIIa administration scheme was proposed (figure 1). Full hematologic preoperative and postoperative data are described in Table 1. Reported total bleeding during the surgery was 50 ml. No short-term or long- term complications were observed up to 24 months of follow- up. The patient continues with ambulatory by the hematology department and 4 years later she was admitted to the oral and maxillofacial surgery service because of gingivorrhagia and dental pain. For that reason, the dental extraction of 5 teeth was scheduled in a single operation time under general anesthesia. To ensure perioperative hemostasis, rFVIIa was used according to the scheme (Figure 2). The procedure ended without bleeding complications with a favorable evolution, for which she was discharged after 48 hours. Currently, the patient continues to be monitored by the hematology and dentistry service for dental prophylaxis.
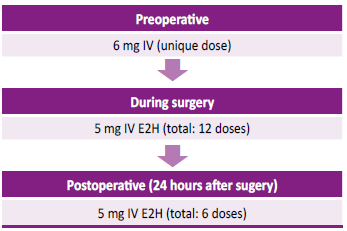
Figure 1.
Therapy with recombinant human factor VII during treatment for abnormal uterine hemorrhage
E2H:everytwohours IV:intravenous
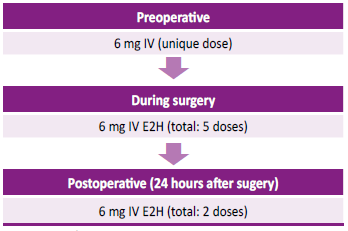
Figure 2.
Therapy with recombinant human factor VII during the treatment for periodontal disease
E2H:everytwohours IV:intravenous
DISCUSSION
GT is a rare disease and both preoperative and surgical management is particularly difficult in affected subjects. Major bleeding and surgeries usually need platelet transfusions, while minor bleeding may be treated with conservative management.[10] Our patient required frequent platelet transfusions during several emergency ward admission, leading to antiplatelet antibodies development. Hysterectomy and salpingo-oophorectomy were proposed to eliminate the known bleeding source and to reduce transfusion requirements.
| Parameter | Preoperative | Postoperative |
| Total leukocytes (cells/ μ1) | 7,110 | 6,800 |
| Hemoglobin (g/dl) | 11.8 | 9.9 |
| Platelets (cells/ μ1) | 213,000 | 174,000 |
| Activated parti al thromboplastin time | 28.62 seconds | |
| Prothrombin time | 9.6 seconds | |
| Fibrinogen (g/l) | 2.44 | |
The administration of rFVII has been recognized as an efficient treatment of bleeding in GT patients with antiplatelet antibodies.[9] In the United States and Canada, rFVII has been approved for patients with GT with refractoriness to platelet transfusions, with or without antibodies to platelets.[1] In our patients, given her refractoriness to platelet transfusions and her low quality of life, rFVII has proposed surgery preparation due to the high bleeding risk during the procedure. Chosen doses were based on available literature; Dardaud et al reported a 55 years-old male with type 1 GT with anti-GP IIb-IIIa iso-immunization, requiring surgery for cholesteatoma. Previously, he had had uneventful surgeries for a neck foreign body and vocal cord nodule removal using platelet concentrates. A few years later, he had been admitted to the hospital for major gastrointestinal bleeding; a colonoscopy was performed to successfully remove two polyps under rFVII infusion (90 µg/kg every 2 h). For elective cholesteatoma operation, after a bolus injection of 90 µg/kg 30 minutes before surgery, rFVIIa was administered in repeated doses of 90 µg/kg every two hours during the first 24 hours, followed by a scheme of 66 µg/kg every 2 h during the next 24 hours and 53 µg/kg every 2 h on the third postoperative day.[9]
In a case reported by Bell et al, a 34 years-old woman had been diagnosed with GT, starting when aged 15 with anaphylactoid reactions to platelet transfusion. HLA-A11 antibodies were detected. In her first pregnancy at age 29 years, type II placenta previa was associated with several antepartum bleeding episodes, all of which required donor platelet transfusions. At delivery, she experienced a major postpartum hemorrhage and two packs of HLA-matched platelets and uterotonic drug infusions were needed. Three years later the patients underwent a lumbar discectomy for an injury following a traffic accident. Even though the surgery was preceded by the infusion of one pack of HLA-matched platelets, a perioperative dural tear was followed by bleeding refractory of local compression and more platelet transfusions. Emergency treatment with 4.8 mg of rFVII was given and bleeding was successfully controlled.[10]
We considered the use of intravenous (IV) rFVII 100 µg/kg (total: 6 mg) prior to the surgery, followed by 84 µg/kg (5 mg) every 2 h up to 24 hours after the surgical procedure. In total, 1 dose of 6 mg and 12 doses of 5 mg were necessary. No consensus has been established in terms of the duration of maintenance treatment, which is defined according to severity of bleeding and the best clinical practice. [2,10] Considering the possible thrombogenic potential of rFVII, a more frequent dose scheme was not recommended. However, in a GT database including 206 surgeries performed in 96 subjects, only one thromboembolic event has been reported.[7]
It is worth noting that access to rFVII in Peru is not immediate; in our patient, several administrative steps were necessary to obtain the appropriate doses before surgery. Our case shows that both early multidisciplinary treatment and drug availability are important for a successful approach of surgery in GT patients, leading to a better quality of life, especially in subjects with refractoriness to platelet infusions. We consider that both the good hematologic and surgical outcomes and the absence of complications after major and minor surgery highlight the importance of our case, considering the low incidence of the disease1 and the lack of national and regional data in Latin America.[5]
REFERENCES
1. Poon M, Di Minno G, Oiron R, Zotz R. New Insights into the Treatment of Glanzmann Thrombasthenia. Transfus Med Rev. 2016; 30(2):92-9 doi: 10.1016/j.tmrv.2016.01.001.
2. Ganapule A, Jain P, Abubacker FN, Korula A, Abraham A, Mammen J, George B, Mathews V, Srivastava A, Viswabandya A. Surgical procedures in patients with Glanzmann's thrombasthenia: case series and literature review. Blood Coagul Fibrinolysis. 2017; 28(2):171-175. doi: 10.1097/MBC.0000000000000524.
3. Toogeh G, Sharifian R, Lak M, Safaee R, Artoni A, Peyvandi F. Presentation and pattern of symptoms in 382 patients with Glanzmann thrombasthenia in Iran. Am J Hematol. 2004; 77(2):198-9. doi: 10.1002/ajh.20159.
4. Martínez-Sánchez L, Quintero-Moreno D. Trombastenia de Glanzmann: conceptos clave de la enfermedad. Revista Cubana de Hematología, Inmunología y Hemoterapia [Internet]. 2019 [citado 13 Jun 2023]; 35 (2) Disponible en: https://revhematologia.sld.cu/ index.php/hih/article/view/993.
5. Valdivieso-Falcón LP, Ajalcriña-Guerrero HR, Contreras-Maldonado R. Tromboastenia de Glanzmann. Paediatrica. 2007; 9(2):70-73. https://sisbib.unmsm.edu.pe/bvrevistas/paediatrica/v09_n2/pdf/ a04v9n2.pdf.
6. Franchini M, Favaloro EJ, Lippi G. Glanzmann thrombasthenia: an update. Clin Chim Acta. 2010; 411(1-2):1-6. doi: 10.1016/j. cca.2009.10.016.
7. Poon MC, d'Oiron R, Zotz RB, Bindslev N, Di Minno MN, Di Minno G; Glanzmann Thrombasthenia Registry Investigators. The international, prospective Glanzmann Thrombasthenia Registry: treatment and outcomes in surgical intervention. Haematologica. 2015; 100(8):1038-44. doi: 10.3324/haematol.2014.121384.
8. Poon MC, D'Oiron R, Von Depka M, Khair K, Négrier C, Karafoulidou A, Huth-Kuehne A, Morfini M; International Data Collection on Recombinant Factor VIIa and Congenital Platelet Disorders Study Group. Prophylactic and therapeutic recombinant factor VIIa administration to patients with Glanzmann's thrombasthenia: results of an international survey. J Thromb Haemost. 2004; 2(7):1096-103. doi: 10.1111/j.1538-7836.2004.00767.x.
9. Dargaud Y, Bordet JC, Trzeciak MC, Vinciguerra C, Negrier C. A case of Glanzmann's thrombasthenia successfully treated with recombinant factor viia during a surgical procedure: observations on the monitoring and the mechanism of action of this drug. Haematologica. 2006; 91(6 Suppl):ECR20.
10. Bell JA, Savidge GF. Glanzmann's thrombasthenia proposed optimal management during surgery and delivery. Clin Appl Thromb Hemost. 2003; 9(2):167-70. doi: 10.1177/107602960300900213.