Reportes de Casos
Harlequin color change in a newborn who was positive for COVID-19: report of a case
Cambio de color arlequín en un neonato positivo a COVID-19: Reporte de caso
Harlequin color change in a newborn who was positive for COVID-19: report of a case
Acta Médica Peruana, vol. 40, núm. 1, pp. 62-65, 2023
Colegio Médico del Perú

Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial 4.0 Internacional.
Recepción: 15 Agosto 2022
Aprobación: 16 Febrero 2023
Abstract: Harlequin color change is a benign, idiopathic, self-limiting disorder characterized by the appearance of skin divided into two distinctly colored areas. Its etiology is unknown but thought to be caused by immaturity of hypothalamic regulation of peripheral vascular tone. COVID-19 infection in neonates is infrequent and rarely symptomatic, with only a few cases described in the literature. In isolation, both conditions have a low incidence. It is the first case reported in the world literature of harlequin color change in a newborn who tested positive for COVID-19. There isn’t a single publication that links harlequin color change to COVID-19.
Keywords: Coronavirus Infections, COVID-19, Newborn, Harlequin syndrome, Pediatrics, Skin Manifestations.
Resumen: El cambio de color arlequín es un trastorno benigno, idiopático y autolimitado que se caracteriza por una apariencia de la piel dividida en dos zonas de color distinto. Su etiología es desconocida, pero se cree que está causada por una inmadurez hipotalámica del tono vascular periférico. La infección por COVID-19 en neonatos es infrecuente y raramente sintomática, con sólo unos pocos casos descritos en la literatura. De forma aislada, ambas afecciones tienen una baja incidencia. Este es el primer caso descrito en la literatura mundial de cambio de coloración arlequín en un recién nacido que dio positivo a COVID-19. Aun no existe ninguna publicación que relacione el cambio de color arlequín con COVID-19.
Palabras clave: Infecciones por Coronavirus, COVID-19, Recién Nacido, Síndrome de Arlequín, Pediatría, Manifestaciones cutáneas .
INTRODUCTION
In 1952, Neligan and Strange first described the Harlequin color change (HCC) as a strange autonomic vascular phenomenon, which is characterized by a sudden, brief change in skin color that appears as if a sharp and straight line is drawn, dividing the body into two hemispheres, presenting erythema on one half and the other pallor [1]. Its true prevalence among newborns is thought to be close to 10% under close observation, and it is listed in textbooks as a “rare” event. However, there is a lot of confusion in the literature about the actual incidence of HCC; Some authors state that it may afflict up to 10% of full-term kids [2–4]. Although its cause is uncertain, it has been linked to hypothalamic functional immaturity [5]. It is frequent in preterm newborns, and only a few cases of term newborns have been reported [6].
SARS-CoV-2 has a similar incubation period in infants as in adults (2-14 days) [7]; however, cases of disease onset immediately after delivery have been reported [8]. COVID-19 infection in newborns is uncommon and infrequently symptomatic, with just a few cases described in the literature. More than 90% of the cases in a Chinese pediatric population study of 2143 individuals varied from asymptomatic to moderate. However, in newborns, the proportion of severe and critical cases was 10.6% [9]. It shows that newborns are more susceptible to severe respiratory failure than anticipated. Another research of 2228 children, newborns, and infants found that 5% had dyspnea or hypoxia, and 0.6% had respiratory distress syndrome or multiorgan failure, with two deaths, one of whom was a newborn with tachycardia, refractory shock, gastric bleeding, multiorgan failure and CID [8], similar to our case. Fever, cough, poor feeding, and diarrhea were the most clinical symptoms reported in neonates infected with the SARS- CoV-2 virus. However, it’s unclear how much SARS-CoV-2 played a role in these symptoms since many of these might be due to other causes such as sepsis, metabolic disorder, transient tachypnea of newborns and neonatal respiratory distress syndrome [10]. We present a case of a newborn with severe symptomatic COVID-19 presenting with the harlequin phenomenon.
CASE REPORT
Newborn of a 34-year-old woman with type 2 obesity complicated by gestational diabetes and mild preeclampsia. Uncomplicated spontaneous vaginal birth at 37 weeks on June 6, 2020. Normal amniotic fluid, 3,785 grams, with an Apgar score of six and eight. At four hours, he experienced progressive respiratory distress with expiratory grunt and cyanosis, Silverman-Anderson (SA) score of four, which led to his admission to the intermediate care unit. The baby was started on intravenous (IV) fluid, antibiotic coverage with amikacin and ampicillin at the standard dose, and given oxygen.
At 20 hours, exacerbation of respiratory distress occurred (SA 6), with signs of low output and vasomotor changes consisting of unilateral hyperemia and simultaneous contralateral pallor with a sharp line dividing the left and right sides of the body affecting the trunk, extremities, and face, a condition known as HCC (Figure 1), this remained for 10 minutes and began to fade on its own until it disappeared completely, only one episode was reported.
At 22 hours, he began to progressively deteriorate; hypoactive with capillary refill time (CRT) greater than 3 seconds, peripheral cyanosis, and SA of six, for which he was admitted to the pediatric intensive care unit (PICU). He underwent endotracheal intubation and was placed on mechanical ventilation (SIMV-PC). Among his ventilator parameters: PIP 38, PEEP: 4, FIO2: 70, FR: 60, T.I: 0.4 seconds. On admission, he presented metabolic acidosis, blood count appeared within normal ranges (Table 1), and negative blood culture.
At four days, poorly reactive with sunken fontanelles, jaundiced up to the legs and arms, bilirubin of 15mg/dl (Kramer: 4), CRT greater than 4 seconds, and hypotensive with mean arterial pressure (MAP) of 20mmhg, requiring the administration of colloidal volume loads (albumin and dopamine). Sepsis markers were found in normal ranges. The imaging study reported a chest X-ray with pneumonia-like bibasal opacities brought on by asymmetric ground glass atelectasis.
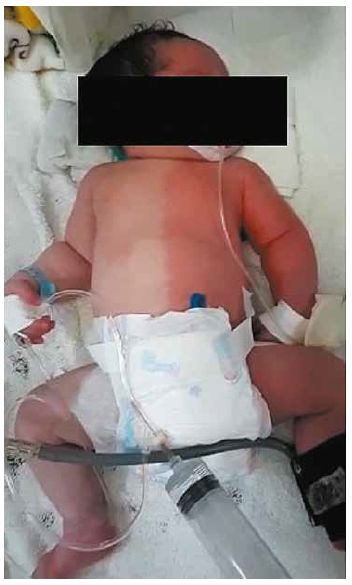
Figure 1.
Harlequin phenomenon in a newborn Regional skin discoloration with sharp edges affecting the face and right and left hemibody of the newborn Regional skin discoloration with sharp edges affecting the face and right and left hemibody of the newborn Selflimited
| Paraclinical report | |||
| Hemogram | 1* | 2* | Normal Values |
| Leukocytes (10^9/L) | 20.3 | 30.9 | 9.1-34 |
| Neutrophils (10^9/L) | 11.9 | 19.5 | 0.1-1.6 |
| Lymphocytes (10^9/L) | 6.4 | 4.4 | 0.8 - 4.0 |
| Hemoglobin (g/dl) | 18.8 | 10.2 | 15-24 |
| Hematocrit (%) | 55.4 | 31.3 | 37.0-54.0 |
| Platelets (10^9/L) | 189 | 25 | 150-500 |
| Arterial BloodGases | |||
| pH | 7.0 | 7.1 | 7.35-7.45 |
| HCO3 (mEq/L) | 9.8 | 9.1 | 19-25 |
| Additional Paraclinics | |||
| Creatinine (mg/dl) | 1.8 | 0.6-1.4 | |
| BUN (mg/dl) | 33.4 | 4.7-23.4 | |
| Urea (mg/dl) | 71 | 10-50 | |
| TPT (s) | 170 | 30-43 | |
| TGO (U/L) | 205 | 0-38 | |
| TGP (U/L) | 184 | 0-41 | |
| Total bilirubin (mg/dl) | 15.8 | 0-1 | |
| Sodium (mEq/L) | 128 | 135-145 | |
At five days, due to ventilator parameters and radiological findings, a rapid antigen test (RAT) for SARS CoV-2 was performed with negative results. In critical condition, hypoactive, weakly reactive, sympathomimetic amine support, CRT more than two seconds, slightly tachycardic (164 bpm), and with decreased air entry.
At six days, the patient evolved to multiorgan failure (SOFA score of 21) involving five systems. Neurological involvement was notorious; no spontaneous mobility, generalized flaccidity, and very poor responses to stimuli. The patient reported disseminated intravascular coagulation (DIC), anemia, thrombocytopenia, partial thromboplastin time (TPT) greater than 3 minutes, oliguria with creatinine of 1.8 mg/dl, transaminases elevated five times over the normal range, hyponatremia and mixed acidosis (Table 2). Packed red blood cells and fresh frozen plasma were transfused repeatedly. Antibiotic coverage was changed to azithromycin and tazocin, which was later changed to levofloxacin due to renal impairment.
At ten days, the patient was hemodynamically stable but with difficulty obtaining an adequate oxygen saturation, reaching a positive end-expiratory pressure (PEEP) of 10 cm and a mean pressure of 26 cm to normalize. These parameters were maintained for 72 hours, after which we could start descents. Abundant secretions were aspirated
At 13 days, the patient was extubated with steroid and diuretic support, remaining with abundant bronchial secretions. Despite mechanical ventilation, kinesiotherapy, continuous nebulization, and third-line antibiotics, he evolved torpidly with multiple changing atelectasis. At 17 days, he was sent to the COVID ward after receiving a positive RT-PCR result for COVID-19.
At 21 days, the patient neurologically persisted with no spontaneous mobility, poor reaction to painful stimuli, and no sucking or swallowing reflex. Due to the poor neurological prognosis, only supportive measures were provided, and he passed away 48 hours later, at the age of 22 days from respiratory failure. The mother and child tested negative for SARS-CoV-2 IgG and IgM antibodies on many occasions.
DISCUSSION
The Harlequin phenomenon differs from other severe conditions in that it does not persist for long and does not result in hemodynamic, respiratory, or neuromuscular abnormalities on its own [1]. This condition mustn’t be overlooked and the diagnosis is made solely based on clinical evidence. [11]
Episodes last between 30 seconds to 20 minutes [12], self-limited [13]. They might occur in a single or numerous episodes. HCC is mainly observed from days two to five after delivery [14], but reported cases happen on the first day of life [11]. The color intensity varies from neonate to neonate and is determined by the severity [15]. Neonates with HCC are in good general condition; it occurs in healthy newborns and in the context of medications, hypoxia, prematurity, and congenital heart disease [3,5]. In our case, our patient had hypoxia. Because it is an uncommon condition, it mustn’t be undiagnosed.
COVID-19 infection in newborns is uncommon and infrequently symptomatic, with just a few cases reported in the literature. In this age range, it is crucial to describe the clinical symptoms and their care [8]. Many newborns infected with COVID-19 have been identified worldwide; nevertheless, this age group's clinical signs and treatment are still being studied. In Honduras, no cases of newborns infected with SARS-CoV-2 have been reported.
CONCLUSION
It is the first ever documented case in worldwide literature of harlequin color change in a neonate positive for COVID-19. Given the low incidence of both entities in isolation and the fact that they were discovered in the same patient, we suggest a strong possibility of SARS-CoV-2 to be the source of the harlequin phenomena in this case and should be considered as an indicator of severe COVID in newborns, but further studies that expand the selection of patients with this condition are needed. There is no literature linking the harlequin phenomenon with COVID-19.
REFERENCES
1. Pinel Guzmán E, Martínez Fernández BJ, Pinel Dubón JR. Harlequin Color Change: a brief literature review. Rev Ciencias Biomédicas. 2021;10[4]:274–80. Available from: https://doi.org/10.32997/ rcb-2021-367.
2. Lee RS, Wan H, Chan RL. Harlequin colour change in a newborn with meningitis. Hong Kong Med J. 2012;18[6]:6–7. Available from: PMID: 23223664.
3. Januário G, Salgado M. The Harlequin phenomenon. J Eur Acad Dermatology Venereol. 2011;25[12]:1381–4. Available from: https://doi.org/10.1111/j.1468-3083.2011.04124.x.
4. Selimoglu MA, Dilmen U, Karakelleoglu C, Bitlisli H, Tunnessen WW, Board A. Picture of the Month. Harlequin Color Change. ARCH PEDIATR ADOLESC MED. 1995;149[10]:1171–2. Available from: https://doi.org/10.1007/978-3-540-69000-9_100031.
5. Garg S, Joshi A, Mehta S. Novel presentation of harlequin phenomenon [simultaneous cyanotic mottling and frightening pallor [vasospasm] of one limb]. J Clin Neonatol. 2019;8[2]:131–2. Available from: https://doi.org/10.4103/jcn.jcn_129_18.
6. Valerio E, Barlotta A, Lorenzon E, Antonazzo L, Cutrone M. Harlequin Color Change: Neonatal Case Series and Brief Literature Review. Am J Perinatol Reports. 2015;5[01]:e73–6. Available from: https:// doi.org/10.1055/s-0035-1545671.
7. Centers for Disease Control and Prevention. COVID-19 Pandemic Planning Scenarios [Internet]. Available from: https://www.cdc. gov/coronavirus/2019-ncov/hcp/planning-scenarios.html#table-2.
8. Panahi L, Amiri M, Pouy S. Clinical Characteristics of COVID-19 Infection in Newborns and Pediatrics: A Systematic Review. Arch Acad Emerg Med. 2020;8[1]:e50. Available from: https://doi. org/10.22037/aaem.v8i1.634.
9. Eastin C, Eastin T. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. J Emerg Med. 2020;58[4]:712–3. Available from: https://doi.org/10.1016/j. jemermed.2020.04.006.
10. Hassan M, Khalil A, Magboul S, Alomari O, Abdalla T, Alsliman H, Alhothi A, Al Maslamani E, AlAmri M, Soliman A. Neonates and Young Infants With COVID-19 Presented With Sepsis-Like Syndrome: A Retrospective Case Controlled Study. Front Pediatr. 2021 Feb 25;9:634844. Available from: https://doi.org/10.3389/ fped.2021.634844.
11. Khemani S, Ali SB, Karim S, Yezdan MA. Harlequin colour change. J Coll Physicians Surg Pakistan. 2017;27[September]:S127–8. Available from: PMID: 28969750.
12. Templet T, Lemoine J. Benign Neonatal Skin Conditions. J Nurse Pract. 2017;13[4]:e199–202. Available from: http://dx.doi. org/10.1016/j.nurpra.2016.09.013.
13. Cizmeci MN, Alagoz D, Avsar MI, Alis G, Tutanc M. Harlequin color change after abdominal paracentesis in a newborn with neonatal hemochromatosis. Pediatr Dermatol. 2014;31[6]:e114–5. Available from: https://doi.org/10.1111/pde.123992014;31[6]:e114–5.
14. Larralde M, Abad ME. Transient Skin Disorders in the Neonate and Young Infant. In: Hoeger P, Kinsler V, Yan A, editors. Harper’s Textbook of Pediatric Dermatology. 4th ed. Wilhelmstift: John Wiley & Sons Ltd.; 2019. p. 72–83. Available from: https://doi. org/10.1002/9781119142812.ch6.
15. Dewangan M. Harlequin colour change in a neonate with perinatal asphyxia: An unusual pattern. J Pediatr Sci. 2016;8:1–3. Available from: https://doi.org/10.17334/jps.10981.